New artificial blood vessel to prevent occlusion and infection
Researchers from SDU and Odense University Hospital aim to develop a revolutionary artificial blood vessel. The EU has backed the project with DKK 40 million.
In a new international research project, researchers from the University of Southern Denmark (SDU) and Odense University Hospital (OUH) seek to develop a revolutionary artificial blood vessel that is prevented from closing or becoming infected.
The problem is prevailing for all artificial blood vessels, but especially when they are used for dialysis.
- This is precisely the reason we are focusing on dialysis patients in our project. If we are successful in developing the new artificial blood vessel, the technology could be used relatively easily and quickly for safe home dialysis and bypass surgery, says Jes S. Lindholt, Professor of Vascular Surgery at SDU and OUH, who is one of the researchers behind the project.
‘Bypass’ means that the surgeons create a detour past a closed area, a kind of orbital road of blood vessels. The potential for the new artificial blood vessels is so great that the EU recently invested approx. DKK 40 million in the project.
New blood vessel to prevent complications
Artificial blood vessels have been used for more than 50 years when the patients themselves do not have the necessary ‘spare parts’.
This may be because the veins cannot be used due to varicose veins, or they are missing because of previous surgery or dialysis access.
Over the years, small advances have been made in the area to prevent the most frequent and serious complications with artificial blood vessels, such as scar tissue formation in the sutures; slow blood flow, which frequently causes them to occlude; and problems with infection around the blood vessel, which can occur even years after they have been implanted.
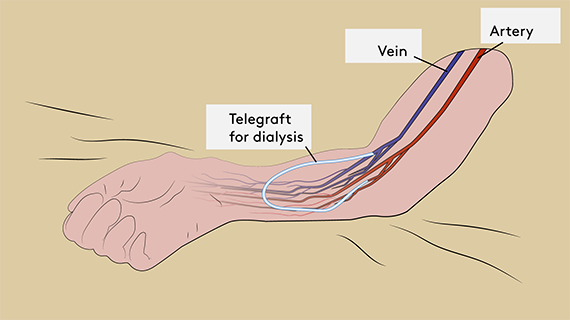
The problems are particularly significant when the artificial blood vessels are used for dialysis, in which case up to 70 percent of blood vessels occlude or become infected within a year.
If they are used for a bypass in the limbs, half of them occlude within five years, and they cannot be used for bypassing small blood vessels such as the coronary arteries of the heart.
- By setting out to develop a solution for the most vulnerable patients – the dialysis patients – who can even use it for safe home dialysis without risking life-threatening bleeding, we are fairly certain that it can also be used for bypass surgery for atherosclerosis within a short time frame. The potential is enormous, Jes S. Lindholt says.
State-of-the-art technology
For a long time, he and Thomas Emil Andersen, who is an Associate Professor at the Department of Clinical Microbiology, SDU and OUH have been working in cooperation with the Danish company Biomodics ApS to develop a special composite material for artificial blood vessels.
The idea is to use a special chemistry that mimics the natural properties of the blood vessels in the body. Medicines can also be embedded in the material to inhibit bacterial infection and scar tissue formation in the sutures.
- Bleeding arising from vascular prostheses is a frequent and life-threatening complication for dialysis patients, Thomas Emil Andersen explains.
- The new technology we are developing in the project inhibits infections by making it more difficult for the bacteria to stick to the material.
Faster and better dialysis
The Swedish company VERIGRAFT contributes technology to improve integration with the surrounding tissue and thereby ensure that the blood vessel quickly merges with its surroundings.
- Combined with a three-layer self-sealing silicone wall in the blood vessel, we expect that dialysis will be safer and can be initiated more rapidly. If all goes well, dialysis patients will also be able to make use of home dialysis, rather than having to spend several hours travelling to and spending time at a dialysis clinic 2-3 times a week, Thomas Emil Andersen continues.
In 2020, according to the National Register of The Danish Society of Nephrology, around 2,600 Danes needed dialysis to remove waste substances and excess fluid from the blood, which is necessary when the kidneys fail.
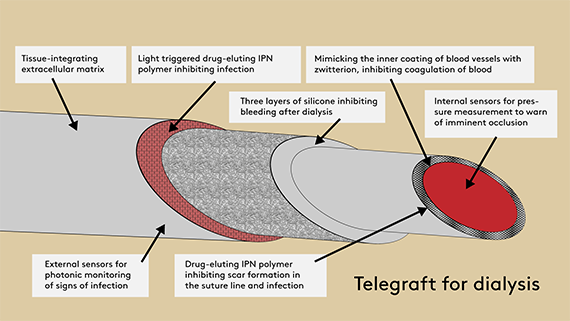
However, the researchers do not believe that the complications can be ruled out completely. For this reason, the blood vessels are equipped with a pressure gauge that can monitor the blood flow remotely, for example from hospitals, and thus send alerts about impending occlusion.
The blood vessels will also have an embedded optical sensor, developed in collaboration with the Leibniz Institute of Photonic Technology, which uses laser technology and so-called Raman spectroscopy to pick up any signs of incipient infection.
- Today we usually only discover any complications when it is too late, but with telemonitoring we get an early warning, allowing us to make corrections before any problems arise. This is a completely new approach which has enormous potential to reduce both patients’ suffering and the costs of the healthcare system, Jes S. Lindholt says.
The upcoming blood vessel is therefore aptly named TELEGRAFT. ‘Tele’ stands for telemonitoring, and ‘graft’ refers to the implantation of the artificial blood vessel.
Once TELEGRAFT is fully developed and has undergone animal experiments, it will be tested in an international study with participants from Denmark, Sweden, Lithuania, Germany and Spain. TELEGRAFT is planned to be ready for clinical use in 4-5 years.
About the project:
The project is called: Telemonitoring of home dialysis utilizing a smart biomimetic arteriovenous graft (TELEGRAFT)
It is funded by:
- EU: NOK 40 million
- The UK Government: DKK 3 million*
- Odense University Hospital: DKK 0.5 million
- University of Southern Denmark: DKK 0.5 million
- Overall budget: DKK 44 million
- Duration: 4 years
*. In support of the English partner at Aston University, which after Brexit could no longer be supported by the EU.
Partners in the project: The University of Southern Denmark (SDU), VERIGRAFT AB (Sweden), BIOMODICS APS (Denmark), THE LEIBNIZ INSTITUTE OF PHOTONIC TECHNOLOGY E.V. (Germany), Odense University Hospital, the Region of Southern Denmark, Aston University (England), BMD SOFTWARE LDA (Portugal), International hospitals involved in the randomised clinical trial: Karolinska Institute (Sweden), ViesojiIstaiga Vilniaus UniversitetoLigonineSantarosKlinikos (Lithuania), The Rechts Der Isar Clinic, The Technical University of Munich (Germany) and Servicio Vasco de Salud Osakidetza (Spain).

Contact:
Jes S. Lindholt, Professor and Chief Vascular Surgeon, Odense University Hospital
Tel.: (+45) 24 64 12 14, email: Jes.sanddal.lindholt@rsyd.dk
Thomas Emil Andersen, Associate Professor and Senior Researcher, Department of Clinical Microbiology, Odense University Hospital.
Tel.: (+45) 21 26 16 34, email: thandersen@health.sdu.dk
Peter Thomsen, CEO, Biomodics ApS.
Tel.: (+45) 61 66 66 19, email: pt@biomodics.com