Lipid-based formulations for drug delivery
Many drugs and especially new promising drug candidates possess poor aqueous solubility thus requiring a suitable formulation approach to facilitate drug administration, improve bioavailability or therapeutic efficiency. Lipid-based formulations, which can be prepared from physiological compounds such as phospholipids and glycerides, offer a promising and very versatile platform for formulations of poorly water-soluble drugs.
My major research interest is in colloidal lipid drug carriers (figure 1A), e.g. particles which are so small that they cannot be seen in a light microscope. Due to the small size, such particles can be injected intravenously. In addition to drug solubilization, nanocarriers may also enable drug targeting, e.g. site-specific drug delivery for example to tumors. However, comprehensive characterization is a prerequisite for the development of carrier systems for drug delivery. In addition to particle size and morphology, stability and drug release characteristics in physiological media are of upmost importance.
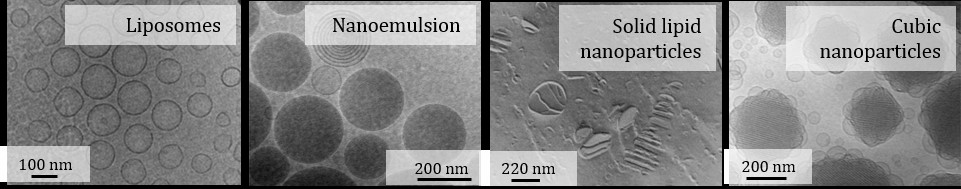
Figure 1: Examples of lipid nanocarriers prepared from polar lipids (liposomes and cubic nanoparticles) and nonpolar lipids (nanoemulsions and solid lipid nanoparticles).
Asymmetrical flow field-flow fractionation (AF4, figure 2A) is a powerful and versatile separation technique covering the whole nm-size range. With the connection of different detectors (MALS, dRI, UV/Vis), this technique enables comprehensive characterization of colloidal dispersions. In addition to accurate size determination (figure 2B), information about particle morphology, co-existing colloidal structures, which are often formed by excess stabilizer in lipid nanoparticle dispersions, and stability and drug release in physiologically relevant media such as serum can be obtained.
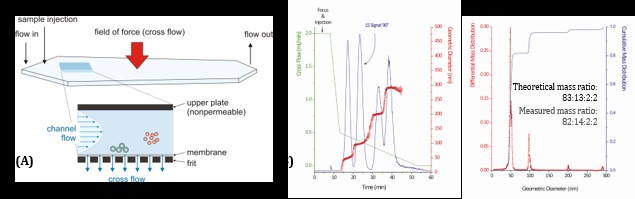
Figure 2: Asymmetrical flow field-flow fractionation. (A) The separation takes place in a thin channel where a cross flow is applied perpendicularly to the parabolic channel flow. Depending on their hydrodynamic size, the particles accumulate at different heights above the membrane where bigger particles are closer to the membrane resulting in a slower movement. (B) Separation and size analysis of a mixture of polystyrene nanospheres (nominal diameters of 50, 100, 200 and 300 nm).
Another research interest is on (trans)dermal drug delivery, where I cooperate with Jonathan Brewer (BMB/SDU). The skin presents an attractive administration route due to the painless and easy drug administration. However, the tight skin barrier presents a considerable challenge, especially when a systemic effect is anticipated (transdermal drug delivery). In addition to physical methods (e.g. microneedles and laser perforation), lipid-based formulations (e.g. deformable vesicles and cubic nanoparticles) can improve drug penetration into the skin. In addition, we are working with organotypic skin models and recently the REK organotypic culture (ROC) has been re-established (figure 3). If reproducible barrier properties are provided, such organotypic models present an attractive alternative to native skin for skin permeation studies and evaluation of new formulations.
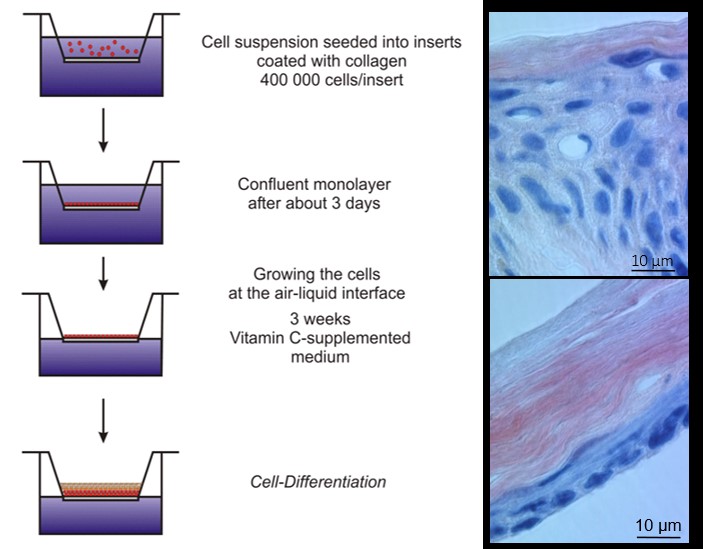
Figure 3: Cultivation of REK organotypic culture (left) and microscopic images of human (top) and ROC (bottom) epidermis.
